Are you getting what you think you’re getting?
January 6, 2020

Discover is fired up for 2021! The New Year is upon us and no doubt you received some amazing products as gifts over the holiday. Have you ever wondered if the product is worth all the hype? Especially with hemp and CBD products flooding the market, how can you be sure that you’re getting what you think you’re getting?
The only way to really know is to check out the product certificate of analysis (COA). We have mentioned before how important a COA is for product and company transparency and it really is the go-to for making sure your product is considered safe by federal standards because consumer safety is the #1 reason why a product undergoes testing. Let’s check out what a COA does and how to read one.
What is a certificate of analysis (COA)?
A COA is a document issued by a laboratory that confirms that a product meets its predetermined specifications. COAs provide critical information to help verify that the company’s product meets or exceeds quality and safety measures. The COA can also be a helpful tool for you to understand what a product contains, or does not contain, and verify that ingredients are compliant with federal and state regulations. Companies who produce the gold standard of cannabinoid products always test for:
- Heavy metals
- Residual solvents
- Pesticides, herbicides, and fungicides
- Microbiological contaminants
- Cannabinoids
Because hemp is what we call a phytoremediator, it essentially cleans the soil it grows in by absorbing everything in the soil. For example, hemp was planted at the site of the 1986 Chernobyl disaster in order to absorb the radiation from the ground.1 This is fantastic for the earth, but when making products for consumption, testing ensures that products are of the highest quality. It is important to test products with methods designed for hemp products, known in the testing world as fit-for-purpose. Companies who want to assure the quality and safety in products they make send their products to be tested by qualified laboratories. Qualified laboratories have ISO17025 certifications. The International Organization for Standardization (ISO) issues various compliance standards and, in particular, the 17025 standard is the globally recognized standard for competence of testing and calibration of testing equipment in laboratories. These ISO-certified labs assure the use of fit-for-purpose methods.
What are heavy metals?
The Food and Drug Administration (FDA) has identified four naturally occurring heavy metal elements, nicknamed “The Big 4” that are commonly found in our environment, and has defined acceptable limits for each of these metals in food and dietary supplements. These are:
- Lead (Pb)
- Mercury (Hg)
- Arsenic (As)
- Cadmium (Cd)
These elements are naturally found in the soil, air, and water, and can be absorbed by plants. Testing ensures that products are not only FDA compliant but also safe for customers!
What are residual solvents?

Solvents are very important for the creation of pharmaceutical and dietary supplement products and are often used at the beginning of the manufacturing process to create the active ingredients for ointments and other topical products. Correct use of these solvents includes proper removal following processing, however if they are not properly removed, residual solvents can compromise both the safety and quality of the product. Residual solvent analysis determines whether solvents such as butane, propane, hexane, and acetone are present in harmful levels in the products you buy.
Why is it important to test for pesticides, herbicides, and fungicides?

In the United States each agricultural crop has a list of approved chemicals and associated maximum levels, or limits, of those chemicals regulated by the Environmental Protection Agency (EPA). Hemp, as of now, has very few approved conventional pesticides for use but is allowed certain biopesticides. Cannabinoid products that are considered the gold standard are analyzed for pesticides (insect-killers), herbicides (weed-killers), and fungicides (fungus-killers) to assure products do not contain these ingredients.
Why test microbiology in each batch?

E. coli, Salmonella, and Listeria are common harmful bacteria that can contaminate food and water. Testing for these bacteria, as well as yeasts and molds, is crucial for the safety of products that are ingested. A sample of each batch is subjected to multiple tests to assure the absence of harmful microorganisms (e.g. bacteria, yeast, or mold) and demonstrate the purity of hemp-derived cannabinoid products.
How Do I Read A COA?
Now that you know what is reported on a COA, how do you read the results? Let’s break an example COA into sections and highlight what we find in each part.
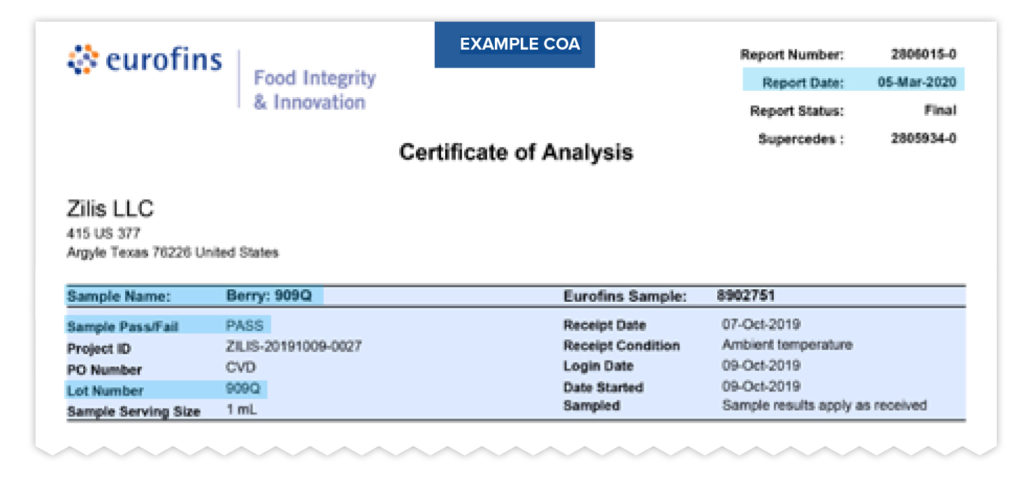
At the top of the COA you’ll find several pieces of identifying information such as the name of the company and which product the COA represents. You will also find other information that legitimizes the COA such as:
- Laboratory name
- Laboratory assigned report number
- Company Name and Address
- Sample name
- Report date
- Product Lot number
Cannabinoid Potency
The next section reports the potency of cannabinoids found in the product sample. Federal regulations require potency information about the THC level that may be found in some hemp products, as well as information about other cannabinoids that are being marketed, such as CBD or CBG. Based on the federal definition of hemp, THC levels cannot be greater than 0.3% by dry weight. As you can see in the example that follows, the THC potency falls well below the threshold allowed by the federal government. To clarify let’s look at the amount of THC in this sample. All full spectrum hemp products will have some amount of THC in them but by knowing how to read a COA, you can see exactly how much THC, along with other marketed cannabinoids, are in your product, and compare that to the label on the product.

Heavy Metals
Another important testing component is the amount of heavy metals that can be detected in the finished product. As described above, the FDA has identified four naturally occurring heavy metal elements and has defined acceptable limits to ensure products are safe for consumers. This section of the COA provides the amount of heavy metals in the product. In this example, you can see that the acceptable limits are far above any detectable limits of heavy metals tested in this sample.

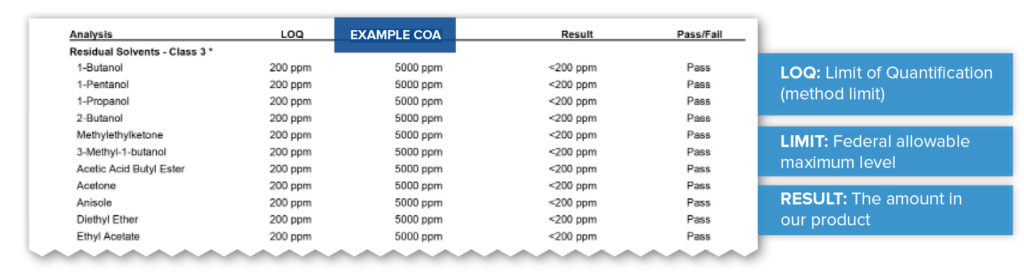
Residual Solvents
The term “solvent” is used to define a group of chemical substances that are used to dissolve or dilute other materials. Testing of residual solvents is performed to make sure that those chemicals, if used throughout the process, are properly removed and do not remain at unsafe levels. In the example below you can see the federally allowable amount versus the amount found in the product. In this case, acetone has an allowable maximum level of 5000 parts per million (ppm). The testing method has a limit of 200 (ppm), meaning a sample has to have at least 200 ppm to register an amount, and here the acetone is so low that it can not be detected and is therefore listed at less than 200ppm.
Pesticides
Pesticides, herbicides, and fungicides can sometimes “drift” from non-hemp crops on nearby fields if grown outside, so it is important to confirm that your product does not contain any of those unwanted or unapproved compounds. In this example, based on federal and state regulations, a maximum level of equal to or less than three-tenths of a milligram per kilogram (<=0.30mg/kg) has been set for specific pesticides. The compounds tested here all “pass” as they come in well below the limit at less than five thousandths of a milligram per kilogram (<0.050mg/kg).
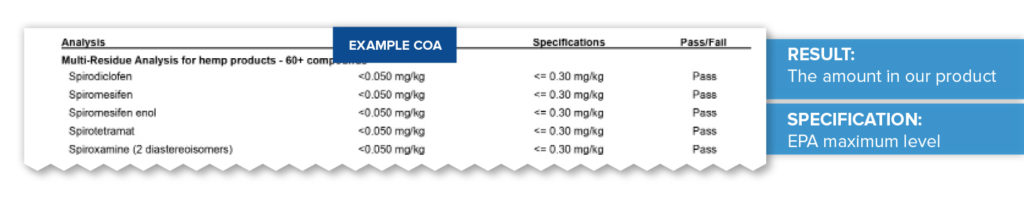
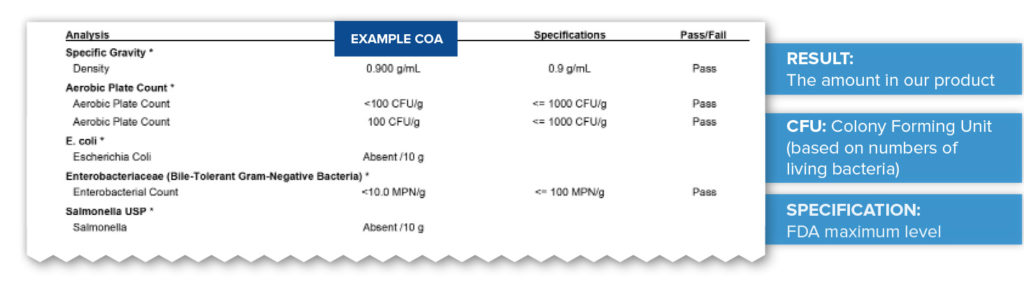
Microbiological Testing
The last important test performed is to check for the possible presence of harmful bacteria. Testing for bacteria is crucial for the safety of products that are ingested. Specific levels set, based on safety data and FDA guidance, for each type of bacteria which are calculated and reported bases on Colony Forming Units (CFU)—the numbers of living bacteria. In the example below you can see that the bacteria E. coli and Salmonella are completely absent. Others are present at levels ten times less than the maximum allowable levels giving this product results that “Pass” strict safety and quality specifications.
Accreditation & Transparency
Finally, if you are looking for the gold standard of products, always check the COA to make sure it was issued by an ISO accredited laboratory and that the laboratory locations are listed for reference on the certificate. The most transparent companies will offer COAs via a QR Code that links to a website or by direct website link. This transparency is an easy way for consumers to identify companies and products that can be trusted.
Understanding how to read a certificate of analysis gives you confidence in knowing the products you buy are:
- Real;
- Safe;
- Being tested (hopefully by ISO 17025 Laboratories); and
- Can be trusted!
Join us next time when we explore the use of cannabinoids in an exploding new market! See you then!
About Zilis’ Scientific Research & Development Department
Our Scientific Research and Development Department is headed up by Dr. Marielle Weintraub, a hemp industry expert. She holds a master’s and a PhD in Behavioral Neuroscience and is very active in many dietary supplement and hemp industry trade associations, including her role as the current President of the U.S. Hemp Authority. Dr. Weintraub is committed to the continued development of hemp-specific information and testing to fulfill the Zilis mission.
Science posts for Discover are co-researched and co-written by Kelly McGill, Senior Scientific Technical Writer at Zilis. Kelly holds a bachelor’s degree in English and a master’s in Linguistics / TESL. She has been writing science-related content for over 20 years and is an expert in making difficult concepts easy to understand.
Zilis is the creator of UltraCell™, a CBD oil product derived from hemp. Based in Argyle, Texas, a suburb of Dallas-Fort Worth, Zilis is privately held. Visit zilis.com for more information.
SHARE THIS POST
ABOUT THIS BLOG
Discover : The blog with the lifestyle, nutrition, science, and history of the hemp industry.
It’s your go-to for the most up-to-date information on hemp, CBD, dietary supplements, and more! Check it out!